Abstract
Introduction: Primary central nervous system lymphoma (PCNSL) is a rare and aggressive B-cell malignancy. Although potentially curable, its prognosis remains dismal. Its treatment is based on high-doses of methotrexate (HD-MTX) and rituximab, followed by consolidation therapy with whole-brain radiotherapy (WB-RT) or autologous stem cell transplantation (ASCT). Currently, there is no consensus about the best consolidation strategy, but better outcomes with ASCT are achieved with conditioning regimens based on thiotepa, a high-cost drug, with restricted use in resource-constrained settings. Additionally, Latin American data on clinical outcomes, prognostic factors, and therapeutic management in PCNSL are virtually unknown.
Methods: This is a retrospective, observational and single-center study, involving 47-Brazilian patients with PCNSL treated at the University of São Paulo from January 2000 to December 2021. We aimed to assess outcomes, determine predictors of survival and compare responses, as well as toxicities in patients consolidated with chemotherapy alone (HIDAC) versus chemotherapy plus WB-RT.
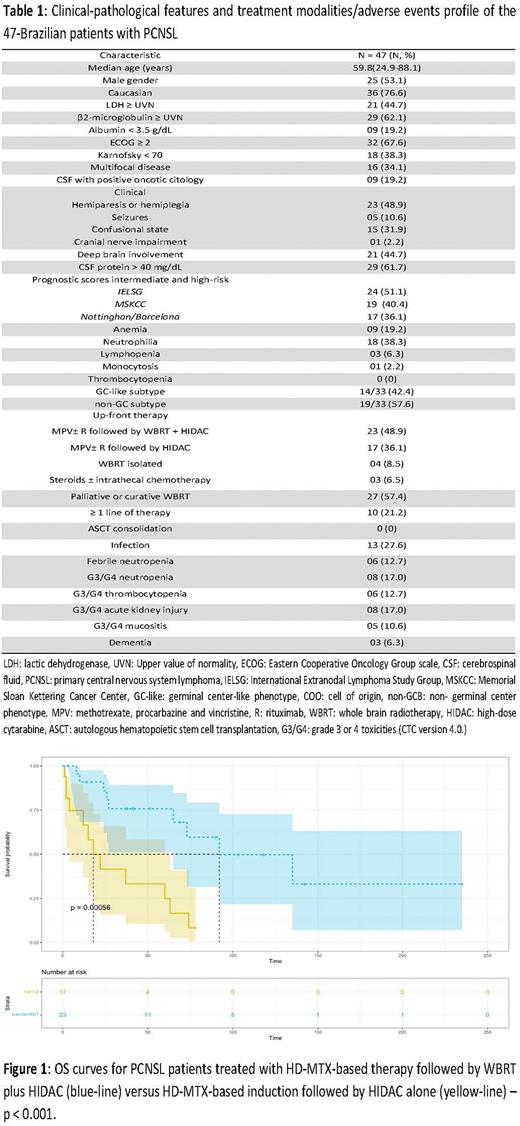
Results: The median age at diagnosis was 59 years (24-88 years), and 53.1% (25/47) were male. LDH ≥ UVN occurred in 44.7% (21/47), ECOG ≥ 2 in 67.6% (32/47), and 34.1% (16/47) had multifocal disease. Hemiparesis was the main clinical presentation, observed in 48.9% (23/47), 51.1% (24/47) had an intermediate or high-risk IELSG prognostic score, and 57.6% (19/33) had an ABC-like phenotype detected by IHC. Up-front therapy involved MPV+/-R followed by WB-RT plus HIDAC in 48.9% (23/47), MPV+/-R followed by HIDAC in 36.1% (17/47), WB-RT alone in 8.5% (4 /47) and steroids +/- intrathecal chemotherapy in 6.5% (3/47). Table 1 summarizes the clinical characteristics and therapeutic approach of the cohort. With a median follow-up of 24.4 months (95% CI: 10.2-41.0), estimated 5-year OS and PFS were 45.5% (95% CI: 32.1-64.7) and 36.4% (95% CI: 23.0-57.1), respectively. Among 40 patients treated with HD-MTX-based induction, estimated 2-year OS was 85.8% (95% CI: 72.2-100) for those consolidated with WB-RT plus HIDAC versus only 41.5% (95% CI: 21.7- 79.7) for those consolidated with HIDAC alone (p<0.001) [Figure 1]. Hematologic and non-hematologic adverse events were not significant, and severe cognitive decline occurred in only 6.3% (3/47) of cases, all of them received WB-RT. In univariate analysis, age < 60 years [HR: 0.46, p=0.05], Hb > 120 g/L [HR 0.84, p=0.06] and consolidation with WB-RT [HR 0.20, p<0.001] were associated with increased OS, however, LDH ≥ UVN [HR 2.03, p=0.02], hypoalbuminemia < 3.5 g/dL [HR 3.39, p=0.009], ECOG ≥ 2 [HR 9.35, p=0.04], Karnofsky < 70 points [HR 2.43, p=0.05] and an Intermediate- or high-risk Barcelona score [HR 4.39, p=0.01] were associated with decreased OS.
Conclusion: Combined consolidation therapy (CCT) based on WB-RT plus HIDAC was associated with increased OS in PCNSL compared to isolated consolidation therapy (ICT) based on HIDAC alone. Here, severe late neurotoxicity was uncommon with this approach. These data support that WB-RT may be an effective and safe alternative to ASCT for consolidation therapy in PCNSL, particularly in resource-constrained settings, where access to thiotepa for pre-ASCT conditioning is not universal.
Disclosures
Rocha:Amgen: Research Funding; Takeda: Speakers Bureau; Pfizer: Speakers Bureau; Amgen: Speakers Bureau. Pereira:Janssen: Research Funding; Zodiac: Honoraria; Astrazeneca: Honoraria, Research Funding; Bayer: Research Funding; Celltrion: Research Funding; Sandoz: Honoraria, Research Funding; Eusa: Honoraria; Takeda: Honoraria; Libbs Farmacêutica: Honoraria, Research Funding; Roche: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal